Frullaniaceae
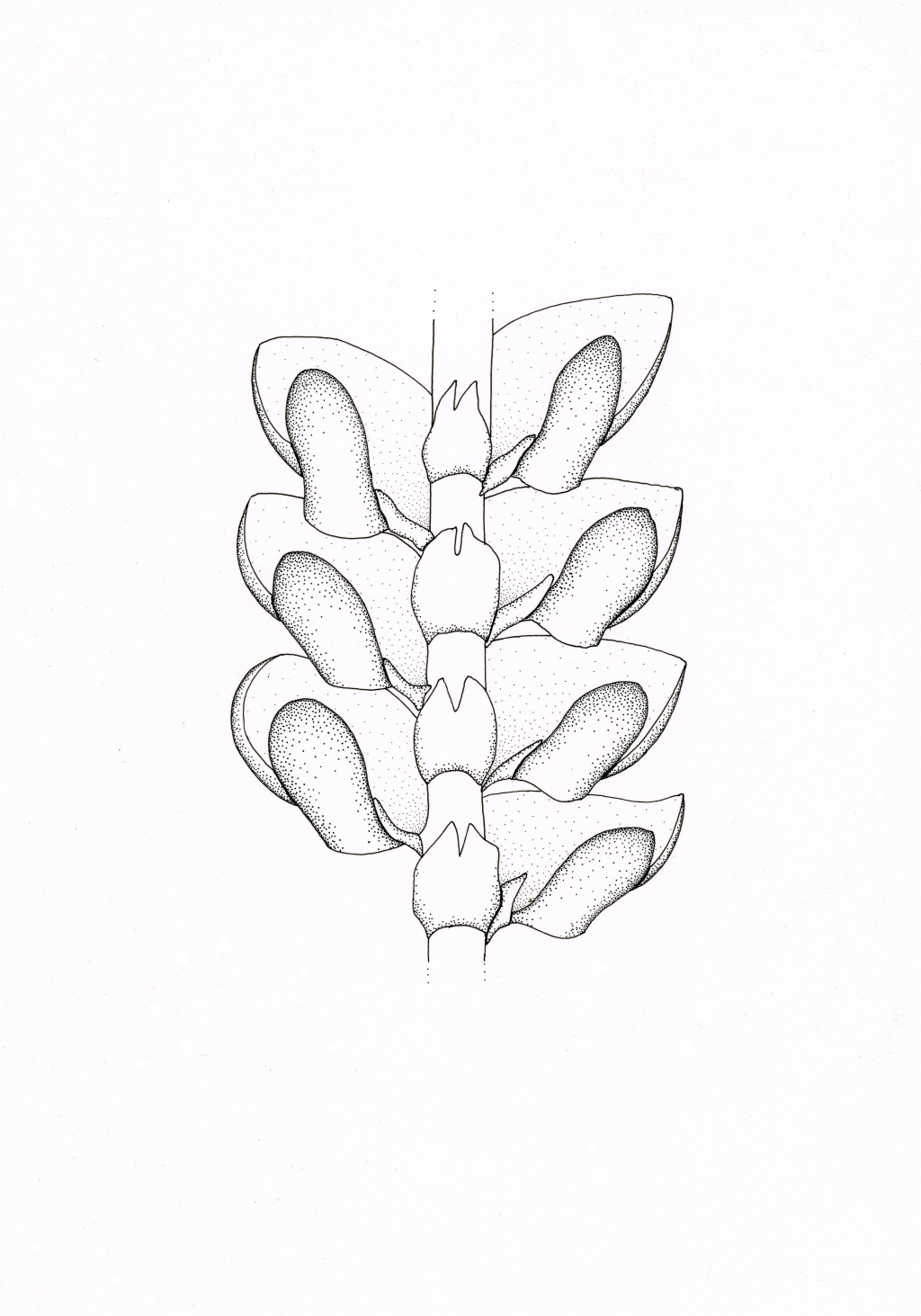
Mostly epiphytic, sometimes lithophytic, monoicous or dioicous, usually closely adhering to substrate, rarely ascending (not in Victoria) or attached only at base and pendent off substrate (not in Victoria). Asexual reproduction occasionally by caducous leaves, caducous tubercles of the perianth surface (not in Victoria) or gemmae from main lobe surface (not in Victoria). Plants often purple, purplish-black or chestnut, with a regularly to irregularly pinnate to tripinnate stem with two ranks of lateral leaves and a single rank of underleaves. Branches emerging from main stem ventral to a lateral leaf lacking lobule and stylus and without a ring of tissue or ring of small leaf like appendages at base. Lateral leaves incubous, 3-lobed, with a smaller abaxial lobule or rarely 2–3 lobules (not in Victoria) and usually with an even smaller abaxial stylus appendage; main lobe not lobed, usually entire, rarely denticulate (not in Victoria) or dentate (not in Victoria), rounded or rarely acute (not in Victoria) or apiculate (not in Victoria) at apex, when moist horizontally spreading or rarely erect from substrate; lobule often saccate and falcate and arched downwards to galeate, erect and taller than wide, or sometimes not inflated and lanceolate, often attached directly to stem, scarcely attached to main lobe or broadly fused to main lobe (not in Victoria), rarely inserted below the main lobe when at the base of a branch (not in Victoria), smooth or rarely papillose (not in Victoria), entire; stylus vestigial, filiform or triangular, attached between lobule and stem. Underleaves usually bilobed, rarely not lobed (not in Victoria), entire or dentate. Leaf cells quadrate, oblate or oblong, thin- to thick-walled, often with intermediate, nodulose thickenings in leaf lobes, lobules and underleaves or sometimes only in the lobules and underleaves, with or without distinct trigones, usually with 2–8 oil bodies, sometimes and particularly toward leaf base with a single large oil body and no chloroplasts (ocelli). Rhizoids in fascicles from underleaf bases, without internal peg-like thickenings, hyaline to brownish. Androecia terminal on lateral branches, of 4–numerous bracts, each covering 2 antheridia. Sporophyte terminal on leading axis or on branch, surrounded by a calyptra and perianth; perianth usually trigonous with a single ventral keel, sometimes with 4 or more keels in addition to lateral keels, rarely without ventral keels, sometimes with teeth (not in Victoria), cilia or tubercles on surface, with a rostrate apex. Seta short, barely elevating capsule beyond perianth. Capsule globose, bistratose, dehiscing by 4 valves; elaters present, unispiral. Spores globose to ellipsoid, with rosette markings scattered regularly over the surface, green, shed singly.
One genus, Frullania, on all continents except Antarctica and with conservative estimates of around 300–375 species (Schuster 1992; Gradstein et al. 2001). Söderström et al. (2016) listed 481 species, which included numerous dubious, poorly known species.
Frullaniaceae is an easily distinguished family of liverworts based on the combination of the chestnut to purplish-black colour of the leaves, the bifid or rarely entire underleaves, and the, entire water sac lobules. These lobules have been suggested to enhance water uptake by increasing the surface area of the leaf (Verdoorn 1930; Puterbaugh et al. 2004) and do not serve as water storage organs because when the leaf dries the water in the sac is removed due to cohesion with water outside the sacs (Blomquist 1929). Occasionally invertebrates, such as rotifers and nematodes, can be found inhabiting these sacs, whose excretions and decomposition could provide supplemental nitrogen to the plant (Puterbaugh et al. 2004). However, the sacs are most frequently free of inhabitants and any possible nutritional benefit to the plant from these organisms will probably be slight and not a key contributor to evolution of this form of lobule (Schuster 1992).
Frullaniaceae has been divided into several subgenera and sections based predominantly on morphology of the lobule and the perianth and associated bracts and bracteoles (Schuster 1992; Hentschel et al. 2009, 2015). Several of these groupings have been shown to form lineages in phylogenies of DNA sequences, supporting their continued recognition, while the morphological features that have been used to define some subgenera (e.g. subgenus Distaloba) have been shown to occur in several separate lineages (Hentschel et al. 2009). Some of these groups are no longer recognised, while some require refinement in their morphological circumscription and taxonomic revision pending incorporation of DNA sequences in phylogenetic studies from a broader sample of species.
In Victoria, the majority of species belong to subgenus Frullania. These species have lobules that are positioned close to the stem, orientated parallel to or slanting back towards the stem. Some distinctive lineages within this subgenus are recognised as sections. Section Australes, comprising species with campanulate lobules, section Acutiloba, comprising species with rostrate lobules with a mucro at the apex, and section Irregulares with perianths with only the two lateral keels, occur in Victoria. Victorian species, which have galeate lobules that are distant from the stem and lean outwards at an angle away from the stem either belong to subgenus Microfrullania (F. rostrata (Hook.f. & Taylor) Gottsche, Lindenb. & Nees) or one of the lineages of subgenus Distaloba (F. aterrima (Hook.f. & Taylor) Gottsche, Lindenb. & Nees).
Frullaniaceae have been well studied biochemically. Some species have been found to produce terpenoids and phenolics with antiseptic and antitumour properties (Asakawa 1998).
Asakawa, Y. (1998). Biologically active compounds from bryophytes. Journal of the Hattori Botanical Laboratory 84: 91–104.
Blomquist, H.L. (1929). The relation of capillary cavities in the Jungermanniaceae to water absorption and storages. Ecology 10: 556–557.
Gradstein, S.R., Churchill, S.P., Salazar-Allen, N. (2001). Guide to the bryophytes of tropical America. Memoirs of the New York Botanical Garden 86: 1–577.
Hentschel, J., von Konrat, M.J., Pócs, T., Schäfer-Verwimp, A., Shaw, A.J., Schneider, H. & Heinrichs, J. (2009). Molecular insights into the phylogeny and subgeneric classification of Frullania Raddi (Frullaniaceae, Porellales). Molecular Phylogenetics and Evolution 52: 142–156.
Hentschel, J., von Konrat, M.J., Söderström, L., Hagborg, A., Larraín, J., Sukkharak, P., Uribe, J. & Zhang, L. (2009). Notes on Early Land Plants Today. 72. Infrageneric classification and new combinations, new names, and new synonyms in Frullania (Marchantiophyta). Phytotaxa 220: 127–142.
Puterbaugh, M.N., Skinner, J.J & Miller, J.M. (2004). A nonrandom pattern of rotifers occupying lobules of the hepatic, Frullania eboracensis. The Bryologist 107: 524–530.
Schuster, R.M. (1992). The hepaticae and anthocerotae of North America east of the hundredth meridian, volume 5. Field Museum of Natural History: Chicago.
Söderström, L., Hagborg, A., von Konrat, M., Bartholomew-Began, S., Bell, D., Briscoe, L., Brown, E., Cargill, D.C., Costa, D.P., Crandall-Stotler, B.J., Cooper, E.D., Dauphin, G., Engel, J.J., Feldberg, K., Glenny, D., Gradstein, S.R., He, X., Heinrichs, J., Hentschel, J., Ilkiu-Borges, A.L., Katagiri, T., Konstantinova, N.A., Larraín, J., Long, D.G., Nebel, M., Pócs, T., Puche, F., Reiner-Drehwald, E., Renner, M.A.M., Sass-Gyarmati, A., Schäfer-Verwimp, A., Moragues, J.S., Stotler, R.E., Sukkharak, P., Thiers, B.M., Uribe, J., Váňa, J., Villarreal, J.C., Wigginton, M., Zhang, L. & Zhu, R. (2016). World checklist of hornworts and liverworts. Phytokeys 59: 1–828.
Verdoorn, F. (1930). Die Frullaniaceae der Indomalesischen Inseln (De Frullaniaceis VII). Annales Bryologici, Supplement 1: 1–187.
 Spinning
Spinning